The Hyve’s Real World Data team recently participated in a collaborative effort to elucidate the best treatment targets for prostate cancer patients. This study-a-thon was part of the IMI PIONEER project and took place in Leiden, The Netherlands from 31st October to 3rd November 2022. The Study-a-thon aimed to identify patients with metastatic hormone-sensitive prostate cancer that were treated with one of the approved treatment plans by conducting observational health data analysis.

The Hyve team had a strong presence in this study-a-thon, with the attendance of Anne van Winzum, Azadeh Tafreshiha, Jan Blom, and Sofia Bazakou. They contributed actively to the event and greatly enjoyed the interaction and collaboration with over 50 other participants. These public and private IMI partners had either gathered in the Astellas offices in Leiden or joined in online - from 14 countries around the world.
As mentioned before, the goal of the event was to generate Real World Evidence seminal to improve the treatment of prostate cancer by enabling better decision-making for healthcare professionals. The results of this effort will be published as a scientific paper.
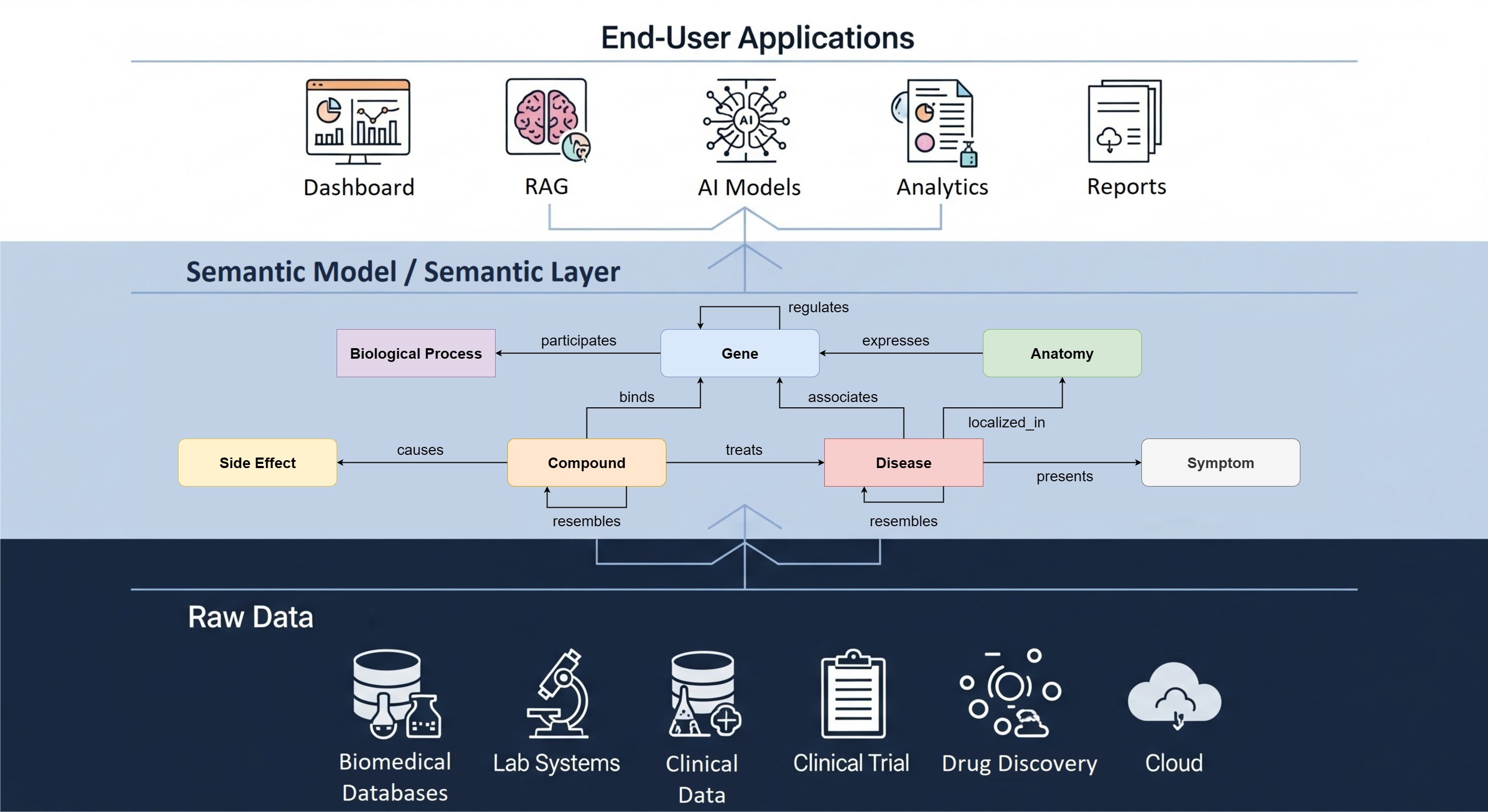
Achieving this goal entails bringing together expertise from various disciplines, from data engineers to physicians. Our representatives from the RWD team have employed their expertise in ATLAS, a unified web interface that attempts to integrate features from various OHDSI applications into a single cohesive experience and in study R packages to fulfill tasks such as phenotype creation, cohort definition, creating new concept sets, and contributing to running the study package.
The PIONEER study is funded through the IMI2 Joint Undertaking and is listed under grant agreement No. 777492. This joint undertaking receives support from the European Union's Horizon 2020 research and innovation program and the European Federation of Pharmaceutical Industries and Associations (EFPIA).