



Psoriasis is a chronic inflammatory skin condition that presents as scaly, painful patches of skin. It can range from mild to severe, and injection treatments that affect the immune system (called biologics) can be prescribed for people who have more severe psoriasis. Biologics are effective at controlling psoriasis, however, individuals with well-controlled psoriasis (clear/nearly clear skin) often need to continue their biologic treatment indefinitely, which can be burdensome. The need for regular injections, frequent hospital follow-ups, and potential side effects, such as infections, can impact a patient's quality of life. Moreover, the cost of long-term biologic treatment for healthcare systems like the NHS is substantial.
Currently, everyone receives the same dose of biologic long-term, however, research indicates that a proportion of individuals can sustain control of their psoriasis with less treatment. If we could tailor (or ‘personalize’) the dose of biologic to each individual, we could reduce the burden and costs of long-term treatment.
The PLAN-psoriasis feasibility trial (isrctn.com/ISRCTN17922845) is investigating whether personalized treatment is acceptable and practical for individuals with psoriasis with well-controlled psoriasis who are receiving biologics, and healthcare professionals. By reducing the treatment burden, personalized treatment may not only improve patient outcomes but also help alleviate some of the financial pressures on healthcare systems.
Participants in the PLAN-psoriasis feasibility trial are randomized to one of three treatment plans for 1 year:
The study is led by St John’s Institute of Dermatology at King’s College London, UK, and opened to recruitment in November 2024. It is being conducted at multiple dermatology centers across the UK. It is funded by the National Institute for Health and Care Research (NIHR) and the National Psoriasis Foundation and also supported by the Psoriasis Association (lead UK charity for individuals affected by psoriasis). A total of 90 participants are being recruited, and the findings will be disseminated through scientific conferences, peer-reviewed journals, and patient networks.
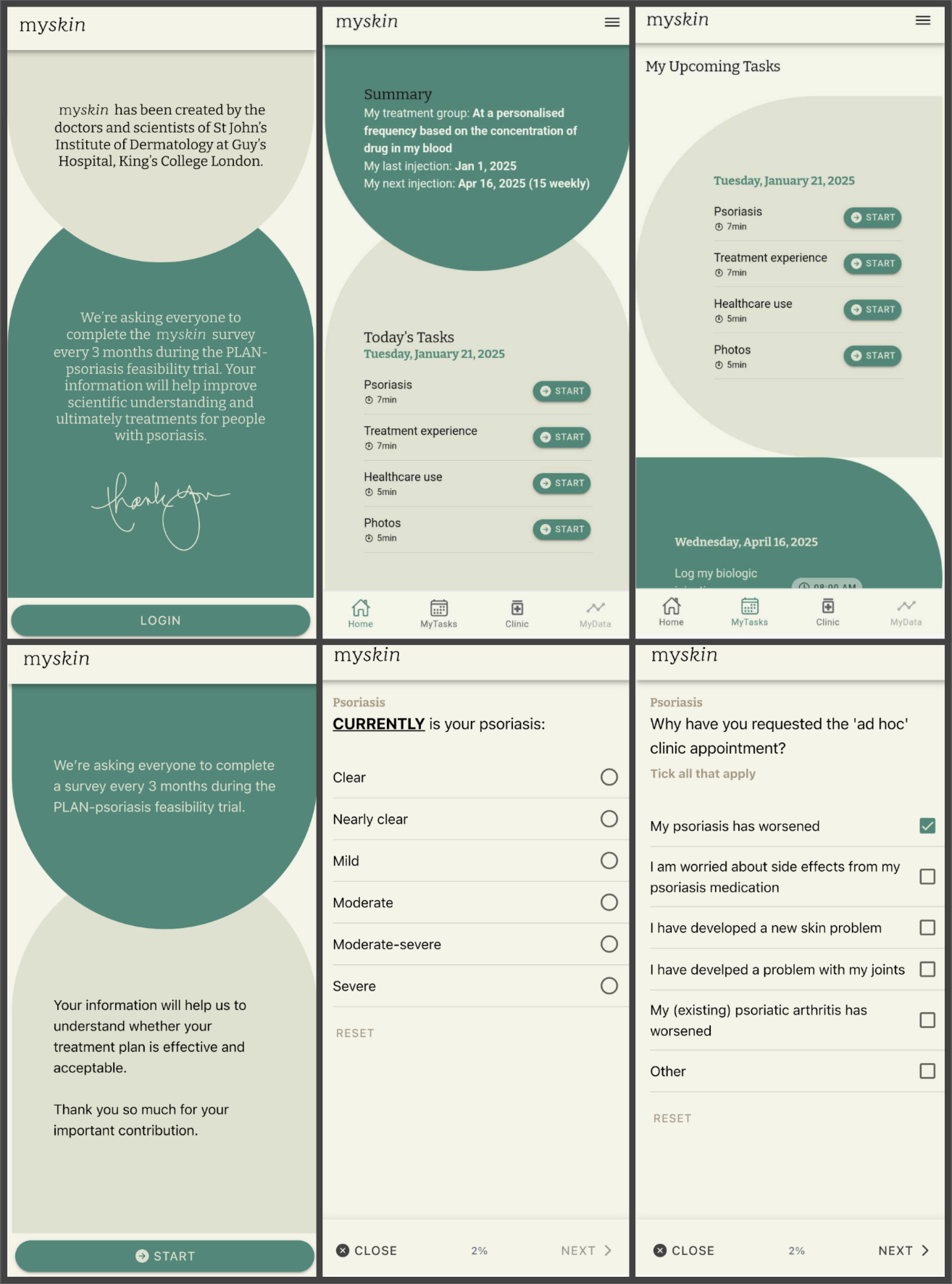
To minimize the healthcare burden for study participants and reduce the need for face-to-face study visits, the team at St John’s Institute of Dermatology sought a digital solution to empower patients to submit patient-reported outcome measures remotely – the ‘mySkin app’.
The mySkin app leverages RADAR-base, a versatile platform for remote monitoring developed by The Hyve, and delivers a tailored solution for the project’s requirements. Key aspects of the solution include:
Throughout the PLAN-psoriasis feasibility trial, participants are asked to complete surveys and submit photographs of their skin every three months using the mySkin app to track changes in their psoriasis. Blood samples will be taken at the start and end of the study, with an additional self-taken blood sample midway through (step-by-step instructions for self-sampling are embedded within the mySkin app). Face-to-face clinic visits take place at the start and end (12 months) of the study and participants have the option to request an in-person ad hoc clinic visit if they feel their psoriasis is worsening or if they have concerns about their treatment. Additionally, a small subset of participants and healthcare professionals will be invited to take part in an optional interview at the study's conclusion to gather insights about their experiences during the study.
The personalized treatment plans in the PLAN-psoriasis feasibility trial represent a promising step forward in the management of psoriasis, offering a more tailored and potentially less burdensome treatment approach. By formally evaluating the feasibility of innovative personalized treatment strategies, the study aims to advance the field toward ultimately improving the lives of people living with psoriasis. The mySkin app is empowering participants to actively monitor and manage their psoriasis while contributing valuable insights to research. By integrating personalized treatment plans and user-friendly digital tools, this project exemplifies a patient-centered approach to chronic disease management. The findings from the PLAN-psoriasis feasibility trial will inform larger studies, driving advancements in personalized healthcare and reducing treatment burden for patients and healthcare systems.
Get in touch to find out if RADAR-base is the platform for you.