The RADAR-base Management Portal is a one-stop shop for managing remote patient monitoring studies. The portal is designed to facilitate easy and secure management of studies and data collected by wearable devices.
The open source software platform RADAR-base was launched in April 2018. The platform enables clinical studies with remote monitoring technologies using sensor data from wearable devices such as smartwatches and Android smartphones. Remote monitoring of patients gives scientists more insight into the health status of patients and enables early detection of relapse, allowing patients and health care personnel to act quickly and adequately.
Planning and managing remote patient monitoring has its challenges. Handling such patient sensitive data requires secure data transfer at all times. At the same time, the privacy of the participants needs to be protected. The Management Portal , therefore, uses pseudonymised data collection. With pseudonymisation, those fields within a record by which a person can be identified are replaced by one or more artificial identifiers. The pseudonymised identifiers assigned to participants and identifiers of their wearable devices should be kept and managed properly to preserve the association and at the same time protect the privacy.
The RADAR-base Management Portal makes planning a new study, managing participants, and adding wearable devices for data collection quick and easy. One important feature is that it supports role based access control. This means that data access is granted or restricted based on the roles and the projects a user is assigned to. This ensures that only authorized personnel can access the research data. Additional security checks are in place to make sure only authorised users can make changes to the database and only verified devices can send data to the platform remotely.
Participant Recruitment
The participant recruitment process is one of the important steps in a remote monitoring study. Medical personnel will recruit eligible patients to a study, provide them with the selected wearable device(s) and give them an Android smartphone or install the RADAR-base apps on the phone they already own. Researcher or the medical personal have the flexibility to choose which device settings are applied. These can be configured in the Management Portal prior to participant recruitment.
Range of wearable devices
Depending on the devices selected for the study, scientists can use various sensors to gather data on heart rate, movement, sleeping patterns, skin temperature, location, et cetera.
The RADAR-base platform currently supports remote monitoring of participants via these wearable devices:
- smartwatches Empatica E4, Pebble 2, and Fitbit Charge HR
- the Bittium Faros, a chest-worn device
- the Biovotion, a smart device worn on the upper arm

RADAR-base can be easily extended to support other devices by developing new plugin software .
Apart from the data collected by the wearable devices, the platform also provides an Android application to support passive data collection using the phone’s sensors. This enables real-time monitoring of movement, location, audio, calls, texts, and app usage. Another Android app is used for active data collection by querying the patients, for example, on their mood, medication intake or the severity of symptoms. Patients are asked to fill in these questionnaires on a regular basis.
The Management Portal also collects low level metadata such as which sensors are used, how often data is collected from a sensor (frequencies) and units of the sensor data. It also allows for customization of these properties across different studies.
Setting up a new study
A new study, called a project on the RADAR-base platform, can only be created by a user with system admin rights. A system admin can view and edit all projects within in the organisation.
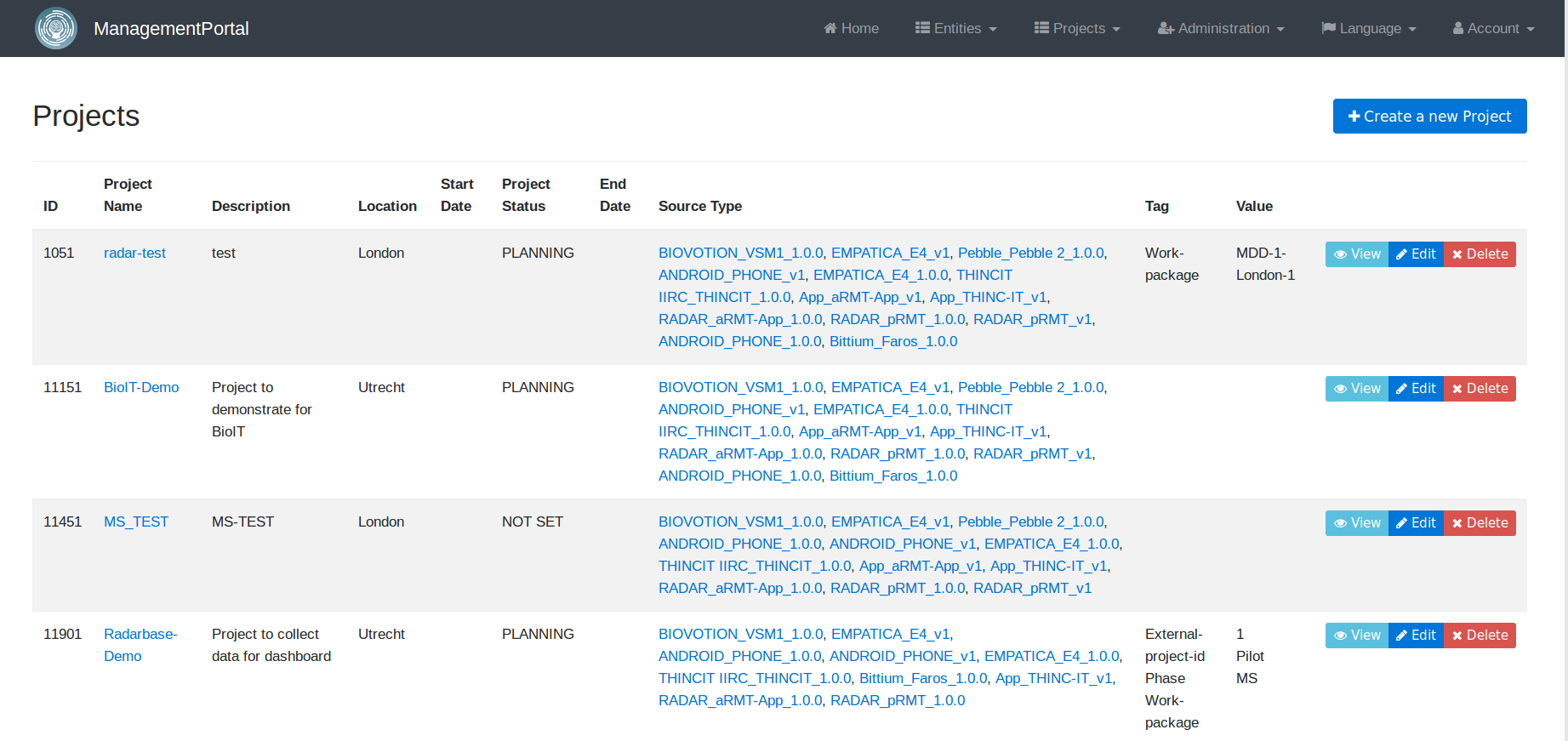
When creating a new project, the system admin is requested to provide details of the study and select the types of wearable devices that need to be supported in the study. New devices can always be added and configured later.

The system admin can also add new users and assign them roles based on projects. The Management Portal gives the project admin(s) an overview of the project, the participants enrolled in the study and the sources linked to each participant – smartphone, apps, and wearable devices.

Device assignment
To each participant, the project admin will assign sources: one or more wearable devices, the smartphone and the apps that will collect patient data. These devices can be assigned to a participant manually or automatically.
Wearable devices that can be easily identified can be linked to the patient account manually. For example, the project admin can assign an Empatica E4 smartwatch can be assigned by entering the device’s ID number.

Other devices such as an Android phones are assigned automatically. This is done through a QR-code generated by the Management portal. Using a QR-code provides ease of use and avoids human and security errors. Once the smartphone with the RADAR-Base app(s) scans the code, it is automatically coupled to the participant. The RADAR-base platform makes sure the data from coupled wearable devices is sent via a secure connection.

Ongoing developments
We are constantly improving the RADAR-base stack and we have recently made a new release of the Management Portal application. The latest version of Management Portal is 0.5.8. Some highlights of the changes from 0.5.3 (the last supported Management Portal on RADAR-Docker) are:
End-user features
- Allows PROJECT_ADMINS to upload data using the newly developed web-application to manually upload data. Watch this space for more news on this.
- We have fixes various bugs. Please check the release notes for details.
- QR Code scanning is made more reliable by making compact QR codes.
Security
- We have upgraded and improved our security packages and token validation mechanism. Watch RADAR-Docker for official support of this version. If you would like to migrate your environments, please read this pull request.
- The Management Portal now provides a ‘oauth/token_key’ endpoint to share public-keys of token verifiers and you can use the latest radar-auth:0.5.8 to automatically make use of this feature.
More information can be found on the official website radar-base.org and detailed documentation is available on the wiki page of RADAR-Base.
Read more on RADAR-Base and RADAR-CNS here.
Please contact us if you want to know how The Hyve can assist to get you started with RADAR-base.



